
|
|
||||||||||||||||
|
Fortune
follows effort!
|
||||||||||||||||
Your
station: |
Home> Knowledge |
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fundamental knowledge: Definitions | Types | Components | History | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lithium batteries | Alkaline batteries | Ni-MH batteries | Lithium ion batteries | | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lead acid batteries | Ni-Cd batteries | Chargers(adaptors) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A battery is a device which can store chemical energy and, on demand, convert it into electrical energy to drive an external circuit. The electrical energy results from a spontaneous chemical energy change(i.e. redox reaction with a negative free energy) within the battery. The redox reagents must not react directly but are consumed at different sites in the battery, at the anode and the cathode, and it is this which causes electrons to flow throuugh the externalcircuit betweenthe battery terminals. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
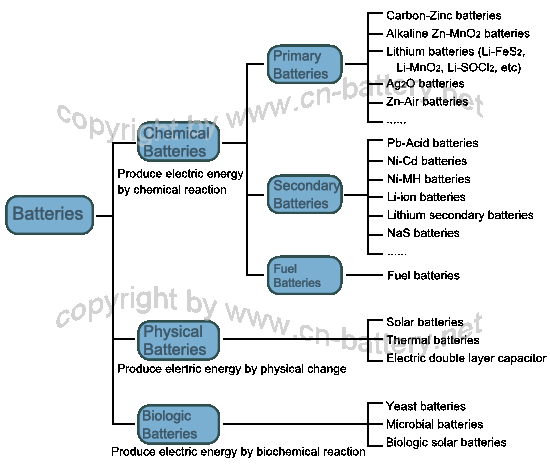 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1. Container The battery case must be resistant to corrosion from both inside and outside, and hence it must be stable to chemical attack by the electrolyte, active mertials and the envirement at the operating temperature. In addition it must have the required mechanical strength, be cheap and light, and there must be a simple method od sealing. This required case is named the container of batteries. 2. Separator The separator must be chemically stable to the electrolyte and to the active materials at the temperature of operation. It is also necessary for the membrane to have the correct qualities of wettability, selectivity, resistivity and flexibility for the particular battery system. 3. Current collector In order for the battery to have an acceptable capacity, the active material is almost always a thick layer of porous, particulate paste, and the electronic conductivity of this material is seldom very high. Hence it is necessary to have a current collector, which is usually a metal grid or sheet, to provide a conducting path through the paste and thereby minimize the resistance of the battery. The current collector also acts as a physical support for the active mass which otherwise would be a very brittle structure. Clearly, the current collector must be stable to chemical attack by both electrolyte and active material, and this limits the choice of material. 4. Electrolyte The selection of electrolyte is determined by the electrode reactions and its concentration is also important. This will control the plate potentials, the electrolyte resistance and viscosity and, by its effect on the rate of diffusion, the differences in concentrations of species between the inside and the outside of the pores of the active paste. Temperature has a large effect on electrolyte properties and both viscosity and resistance increase by more an order of magnitude as the temperature drops from abient to -30°C. This largely accounts for the poorer performance of the battery at lower remperature. The weight of the electrolye is a major contribution to that of the complete battery and hence must be minimized. In any case the electrode spacings should be small to minimize battery resistance. 5. Active materials For a battery with a reasonable discharge rate and capacity, the electroactive species must be readily available at the sites of electron transfer and be present in large quantities. In most batteries these necessities are provided by using solid reactants, and at least in secondary batteries the product of the electrode process is also solid; the anion of electrolyte and sometimes the proton are also participants in the chemical change during the charge or discharge process. Hence if the change is to be accomplished at a reasonable rate, intimate contact and a high-area surface between the solid reactants and electrolyte are essential. This is accomplished by using the electroactive materials in the form of a paste on the current collector. The sizes of the particles and the pores, i.e. the porosity of the paste, are important in determining the performance of the battery. In practice, porosity should be about 50%. Below this value, the unilization of the active material suffers due to pore blocking, and above it the mechanical stability becomes a problem. Hence there is a trade-off between capacity and cycle life. The loading of the paste, the thickness of the porous layer, also affects performance. Thin plates improve capacity particularly at high discharge rates and also give a higjer power density. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||